Water is an “unusual” substance. Not “unusual” like a thoughtful Republican, or living Dinosaur. Unusual in that it has properties that most other kinds of matter don’t have. The most obvious example is that when water freezes it becomes less dene than the unfrozen variety. This leads to icebergs and cocktails: water floating on itself. In fact, the densest water is at 39° F. So in winter the water at the bottom of a pond is often a bit warmer than that at the top. Water also requires a lot of energy to change its temperature: it has a high specific heat capacity.
This can be observed by measuring the temperature of a quantity of water while one applies heat. To illustrate, I’m going to perform an experiment in the confines of my very own home. I’m going to use a teapot as my “quantity of water” holder. For lack of a better implement, I’m going to use the kitchen stove as my heat producer. Water boils at 100 degrees Celsius or 212 Fahrenheit at sea level.
After placing the kettle on the stove, I turned on the heat to “high.” You’ll have to bear with the technical terms. To measure the temperature I’m using a thermometer (again, sorry about the terminology). I made some errors and had to switch thermometers as the first was measuring the temperature of the metal and not the water, but once I got that ironed out (a little joke) all went well. On the chart below you can see that I’ve plotted my data vs time on the X axis (the righty-lefty-one) and the temperature on the y-axis (the uppy-downy-one). I hope you’ll immediately notice something. The temperature plateaus at about 212°. This state is called boiling, you’ve probably heard of it – it’s featured in a lot of recipes (make pasta, make tea, make ramen). The water doesn’t get hotter as it’s boiling.
For the second part of the experiment I’m going to use my imagination. In this case I’m going to add another common liquid to my teapot and add heat. I could use one of the household cooking oils, but they’re kind of expensive. Instead, I’ve decided to use gasoline. It’s cheaper than any of the other liquids in the house (that must be because we have to dig it out of the ground, transport it thousands of miles, refine it from oil, fight wars over it and keep it in special containers because it is so reactive). Now gasoline is great for many things – well really only one, running engines, but it is not great in the kitchen. Even in my imagination I am forced to ignore the boss’s scorn. Two things should be noted: the gasoline will heat up more quickly, and we will be buying a new kettle.
If I were to take any of those more expensive household liquids: detergent, Dr. Braunner’s, Olive Oil, Vegetable oil, Vodka, Coconut oil, I’d find that most would also heat up more quickly and boil at a lower temperature than water. Vinegar might be an exception because it’s really mostly water – it’s also cheaper than gasoline but just barely (3 dollars a gallon). Water has a high “specific heat” that is, it takes a lot of energy to raise a given amount of water a degree Celsius.
Water also has to lose a lot of energy to freeze and once frozen, takes a lot of energy to melt. Hence we’ve got blocks of ice sitting in my driveway on a 50 degree day in January.
Water is an “unusual” substance. Not “unusual” like a thoughtful Republican, or living Dinosaur. Unusual in that it has properties that most other kinds of matter don’t have. The most obvious example is that when water freezes it becomes less dene than the unfrozen variety. This leads to icebergs and cocktails: water floating on itself. In fact, the densest water is at 39° F. So in winter the water at the bottom of a pond is often a bit warmer than that at the top. Water also requires a lot of energy to change its temperature: it has a high specific heat capacity.
This can be observed by measuring the temperature of a quantity of water while one applies heat. To illustrate, I’m going to perform an experiment in the confines of my very own home. I’m going to use a teapot as my “quantity of water” holder. For lack of a better implement, I’m going to use the kitchen stove as my heat producer. Water boils at 100 degrees Celsius or 212 Fahrenheit at sea level.
After placing the kettle on the stove, I turned on the heat to “high.” You’ll have to bear with the technical terms. To measure the temperature I’m using a thermometer (again, sorry about the terminology). I made some errors and had to switch thermometers as the first was measuring the temperature of the metal and not the water, but once I got that ironed out (a little joke) all went well. On the chart below you can see that I’ve plotted my data vs time on the X axis (the righty-lefty-one) and the temperature on the y-axis (the uppy-downy-one). I hope you’ll immediately notice something. The temperature plateaus at about 212°. This state is called boiling, you’ve probably heard of it – it’s featured in a lot of recipes (make pasta, make tea, make ramen). The water doesn’t get hotter as it’s boiling.
For the second part of the experiment I’m going to use my imagination. In this case I’m going to add another common liquid to my teapot and add heat. I could use one of the household cooking oils, but they’re kind of expensive. Instead, I’ve decided to use gasoline. It’s cheaper than any of the other liquids in the house (that must be because we have to dig it out of the ground, transport it thousands of miles, refine it from oil, fight wars over it and keep it in special containers because it is so reactive). Now gasoline is great for many things – well really only one, running engines, but it is not great in the kitchen. Even in my imagination I am forced to ignore the boss’s scorn. Two things should be noted: the gasoline will heat up more quickly, and we will be buying a new kettle.
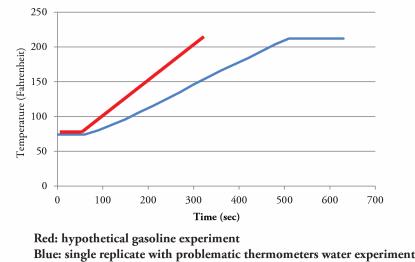
If I were to take any of those more expensive household liquids: detergent, Dr. Braunner’s, Olive Oil, Vegetable oil, Vodka, Coconut oil, I’d find that most would also heat up more quickly and boil at a lower temperature than water. Vinegar might be an exception because it’s really mostly water – it’s also cheaper than gasoline but just barely (3 dollars a gallon). Water has a high “specific heat” that is, it takes a lot of energy to raise a given amount of water a degree Celsius.
Water also has to lose a lot of energy to freeze and once frozen, takes a lot of energy to melt. Hence we’ve got blocks of ice sitting in my driveway on a 50 degree day in January.
Related


